Table 4 shows data for the titration of a 250-mL sample of 0100 M hydrochloric acid with 0100 M sodium hydroxide. C If all the water remained in the gas phase the partial pressure of water at a total pressure of 200 atm would be PH 2O Ptotal mol fraction H 2 O 200 atm 012 240 atm However the partial pressure of water cannot be greater than 0197 atm and the excess will condense.

Autoionization Of Water Educational Infographic Video Anatomia Apuntes Sana
The values of the pH measured after successive additions of small amounts of NaOH are listed in the first column of this table and are graphed in Figure 1 in a form that is called a.

. Complete Solutions Manual General Chemistry Ninth Edition. Academiaedu is a platform for academics to share research papers. The simplest acid-base reactions are those of a strong acid with a strong base.
Hydroxide is a diatomic anion with chemical formula OH It consists of an oxygen and hydrogen atom held together by a single covalent bond and carries a negative electric chargeIt is an important but usually minor constituent of waterIt functions as a base a ligand a nucleophile and a catalystThe hydroxide ion forms salts some of which dissociate in aqueous solution. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon.
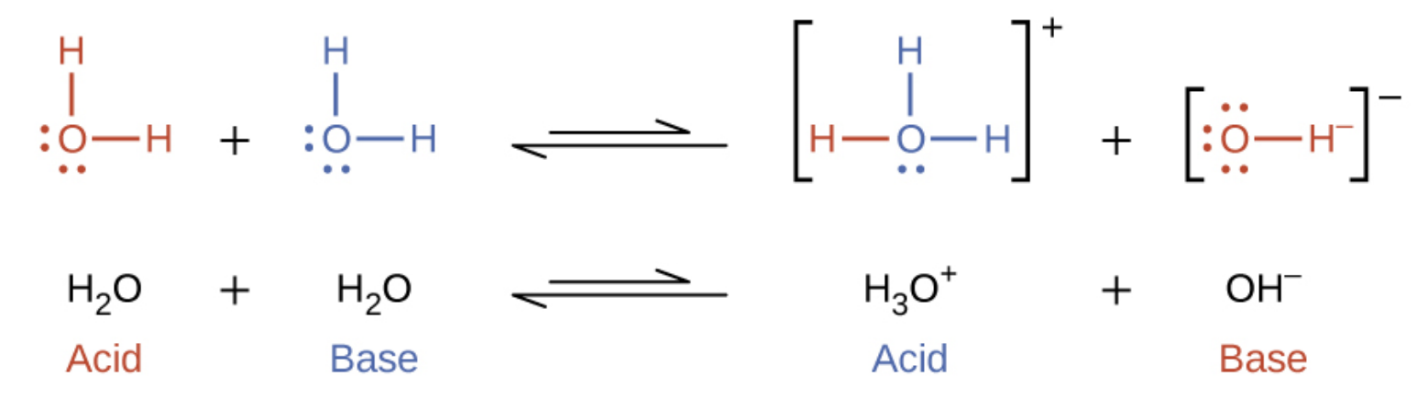
5 2 Autoionization Of Water Ph Poh General Chemistry For Gee Gees
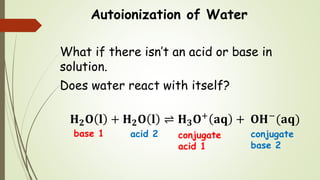
Chem 2 Acid Base Equilibria Ii The Auto Ionization Of Water

Solved A Write A Chemical Equation That Illustrates The Autoionization Of Water B Write The Expression For The Ionproduct Constant For Water K W Mathbf C If A Solution Is Described As Basic Which Of
0 Comments